Why would you want to learn more about some of the ingredients that go into all natural personal care products? Why would you want to have more of a background about fatty acids and lipids in nutrition and every day life? Simple. Understanding the basics of the different types of fatty acids (oils and fats) will allow a person to make healthier choices in both their skin care and nutritional needs.
The goal here is only intended to provide the basics, not to be the comprehensive guide to fatty acids. Warning: there is a little bit of chemistry in this but nothing too painful! If you enjoy chemistry, this will be no problem, and if you hate chemistry, you may want to scroll past the images and get to the bottom line points. But the chemistry is important in understanding why fatty acids have important differences in their properties, and what that means to you.
As reference sources, much of the information in this article consists of my summaries of the relevant sections of several college text books listed at the end of this article. All images have credits listed or are from Wikipedia.
Introduction
The term “lipid” is a slightly technical chemistry and biology term that indicates substances that include oils and fats, and also some other compounds such as waxes, sterols (e.g. cholesterol), steroids, phospholipids, and fat soluble vitamins. This family of compounds, commonly just referred to as “lipids”, shares that they are hydrophobic (not soluble in water), and they are primarily comprised of carbon and hydrogen. The main difference between an “oil” and a “fat” is simply that the former is a liquid at room temperature, while the latter is a solid at room temperature. Most “butters” would also be considered “fats” since they are more or less solid at room temperature, even though they will get soft in warmer “room temperatures”. To be technically accurate, the scientific definition of “room temperature” is about 22 degrees C or about 72 degrees F.
Structure and Characteristics of Fatty Acids (FAs)
Fatty Acids, often abbreviated as just “FAs” are found in many foods such as nuts and seeds, vegetable oils and some grains. As the name implies, fatty acids structurally appear acidic due to the carboxylic acid end (a brief explanation of this later). However, because pH is a measure of acidity or alkalinity in an aqueous (water) solution, there is technically no pH value for pure fatty acids. The pH of these can only be measured once they have been mixed with some amount of water, for example in a personal care product or a food item.
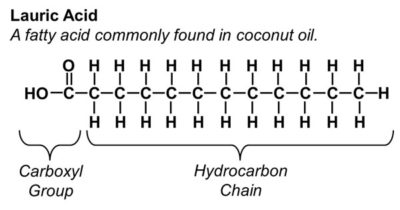
FAs have a hydrocarbon structure – primarily a chain of linked carbon atoms as a backbone (the “C” are the carbons in the diagrams) with hydrogen atoms linked off of the carbons (the “H”s are the hydrogens in the diagrams). They can be diagrammed something like this:
Figure 1. An example of a FA – specifically a saturated FA. More specifically, Lauric acid. Note the carboxyl group is what makes it potentially acidic.
The most common sources of hyrdrocarbons are from oils and fats from various plants and animals, and from petroleum. For the purpose of this article I will mostly focus on oils and fats from plants, not from petroleum or animals. However it is worth pointing out that many of the principles that apply to plant FAs also apply to animal FAs, and also, but to a lesser degree to petroleum products. It is also worth pointing out that these similarities in certain aspects (but with important differences) allow manufacturers of personal care products to get away with using petroleum based ingredients in their products even though that might not be the healthiest choice for your skin. We explore some of this in our articles on chemical preservatives (part I and part II). Further elaboration of how petroleum ingredients are often used in skin care products, and why they are not the best choice, will be the topic of a future article.
Two of the most important characteristics that identify FAs and give them their functional properties are the length of their carbon chains and the number and position of carbon=carbon double bonds. Essentially the length of the carbon chain combined with the number and positions of carbon double bonds identifies and defines all FAs and their properties. For those not familiar, each periodic table element “prefers” to have a certain number of chemical bonds. Carbon happens to “prefer” having four bonds. So any time there are carbon atoms in a hydrocarbon chain that are missing hydrogen (or other bonded molecules), they will form a second bond with their neighbor, called appropriately enough, a double bond. FAs with double bonds can be diagrammed something like this (where the “C=C” are):
Figure 2. Two FAs – one shown with a “trans” configuration, and one with a “cis” configuration.
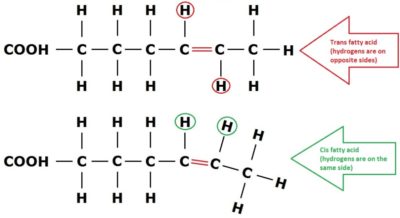
Image courtesy: http://katherineau.com/wp/index.php/2015/08/26/all-about-fat-and-the-saturated-fat-controversy/
FAs that have no carbon double bonds are “saturated” with hydrogen, thus where the term “saturated fats” comes from. Since carbons that are saturated with hydrogen already have four bonds, they do not need to form double bonds with their neighbors. Figure 1 above shows an example of a saturated FA. FAs with carbon double bonds are “unsaturated” because they are not full with hydrogen. However as I will cover shortly, there are several types of unsaturated FAs. Figure 2 shows examples of unsaturated FAs with carbon double bonds.
FAs can have different numbers of carbon double bonds. FAs that have multiple carbon double bonds are known, as “polyunsaturated” FAs (“poly” meaning many). FAs with one carbon double bond are known as “monounsaturated” FAs (“mono” meaning one). And as mentioned, FAs with no carbon double bonds are known as “saturated” FAs. There are more than one type of FA in each of these categories, which I explain below.
Regarding the length of a carbon chain, from a nutritional (or energy) standpoint, longer carbon chains contain more energy and shorter carbon chains contain less energy. Double bonds in a hydrocarbon chain also reduce the amount of energy it contains. So fully saturated fats contain more energy or calories (kcals) than unsaturated fats, assuming equal length. The length of a hydrocarbon chain will also affect other properties, such as viscosity or how “thick” the FA is when liquid, and solubility or how well it dissolves and what will dissolve into it. All things being equal, longer FAs will gel (solidify) at higher temperatures, and shorter FAs will gel (solidify) at lower temperatures. So length can affect the properties or characteristics of FAs. An example of a long FA is oleic acid (18 carbons long), found in olive oil, which is why olive oil tends to gel in the fridge. An example of a short FA is caprylic acid, found in milk fat and coconut oil, which is why these butters tend to get soft at room temperature compared to say animal lard, despite the fact that both types of fats are saturated.
Carbon double bonds also play important roles in the properties or characteristics of FAs. One important effect that carbon double bonds have is that they change the shape of the FA by causing bends in the carbon chain. Therefore, the location and number of the carbon double bond(s) changes the shape of each FA, making each FA unique and having unique properties as a result. In chemistry and biology, shape (or “conformation”) is extremely important in terms of how molecules behave, so these subtle changes in shape due to carbon double bonds can have a large impact in how FAs behave in chemical or biological systems. Or to put it simply, these subtle changes can have a huge impact on how these FAs will react on your skin, or in your body.
For example, Figure 2 shows the difference between a FA with a carbon double bond with a naturally occurring “cis” form (hydrogens present or absent on the same side), versus a FA with a carbon double bond with an artificially created “trans” form (hydrogens present or absent on opposite sides). Note that while they both have bends due to the double bond, the shape is quite different, and the “trans” FA is much straighter, while the “cis” FA is more curved. As a reminder, Figure 2 is again shown below:

In human nutrition, this difference in shape makes the difference between a FA that is an essential nutrient (the cis FA) and one that causes various pathologies (the trans FA – as in the infamous “trans-fats”). It is also worth pointing out that saturated fats, such as pictured in Figure 1, are also quite straight, but do not necessarily pose the same health risks that trans-fats do. Subtle differences in shape can have large impacts in function.
The relative degree of how straight or curved a FA is, also affects its melting point. Or to state it another way, this affects whether a particular FA is a solid or a liquid at room temperature, the temperature at which it will melt, or if it becomes a solid in the fridge or freezer. For example, coconut “oil” is usually a solid butter at standard “room temperature” of 72 degrees F (unless fractionated), whereas olive oil is a liquid at standard room temperature. But if you put olive oil in the fridge it gels or almost solidifies. On the other hand, flax oil will remain liquid, both at room temperature and in the fridge. These differences are determined by the number of carbon double bonds contained in the predominant FAs in each oil. In fact, it is possible to predict to a certain extent what the melting point and certain physical properties of an oil will be based upon the structure of the FAs it contains. (This is also true of oils used for lubrication purposes, including petroleum oils.)
The more saturated a FA is, the straighter it will be. A straighter shape allows the individual FA (or mono, di- or tri-glycerides) to pack together more closely which is what makes a FA a solid instead of a liquid. The more carbon double bonds a FA has (the less saturated it is), the more bends it will have, and this forces the molecules to be spaced further apart. Basically more carbon double bonds lead to less densely packed FAs, and therefore more likely it will remain a liquid at a given temperature.
Since both carbon double bonds and carbon chain length affect the melting point of a FA, which has more impact? Based on many real world examples, it seems that carbon double bonds tend to have more of an effect on melting point than carbon chain length, thus why mostly monounsaturated olive oil is liquid at room temperature (despite containing long carbon chains), while mostly saturated coconut oil remains a solid (despite containing short carbon chains).
Carbon double bonds also make FAs more susceptible to oxidation or rancidity. So the more carbon double bonds a FA has the less chemically stable it is and the more likely it is to go rancid through oxidation. Conversely, more saturation makes a FA more stable, and less prone to oxidation and rancidity. This is why prior to the invention of “hydrogenated” or “trans” fats, saturated fats where used in processed food products that needed to have long shelf lives. This was actually a large part of the reason for the invention of hydrogenated trans fats – they are also very stable and resistant to rancidity. But just because something lasts longer, doesn’t mean it’s beneficial or healthy for you. If you would like more explanation of rancidity, Wikipedia provides a brief explanation here. While saturation of a FAs makes them more stable and resistant to oxidation, it does not necessarily increase the temperatures at which they smoke or burn. The flashpoints of FA also seems to be mostly unrelated to saturation and double bonds. A list of smoke points of various oils can be found here, and an additional list of flash points and fire points can be found here.
To briefly clarify, a monoglyceride, diglyceride, or triglyceride, just refers to one, two, or three FAs being bound to a glycerol molecule, which is a common method for organisms to store FAs. (Think of the glycerol that is used in many soaps – that occurs naturally in most plants and animals.) Oils and fats can have a mix of individual FAs known as free FAs, monoglycerides, diglycerides, or triglycerides. These are merely differences of how the plant or animal stored the FAs at a given time and do not have as much of an effect on FA properties as the other topics discussed here. The health implications of fatty acids are mostly determined by their double bonds and carbon chain length, not by whether they are in the form of mono, di, or triglyceride, or free fatty acid. Here is a diagram of three saturated FAs bound to a glycerol molecule to form a triglyceride. (This diagram uses a slightly different “shorthand” format.)
Figure 3. A triglyceride with the glycerol and FA portions color coded.
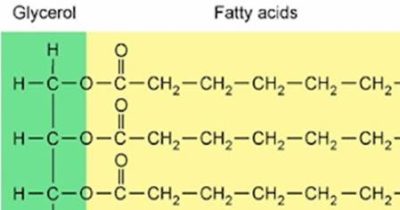
Image courtesy of: https://biochemicalblogger.wordpress.com/2013/04/13/molecules-gone-wild-youtube-review-2/
Similarly clarification is needed for naming conventions of FAs. FAs generally have two ends – one end is called a “carboxy end” and the other is called “methyl end”, and the naming depends on counting the number of carbons to the first double bond from the correct end. (If you are not a chemist then it is not important to the rest of the article for you to know what a carboxy or methyl are, and if you are a chemist you already know what they mean.) If counting from the carboxy end the “delta” naming convention is used, if counting from the methyl end the “omega” naming convention is used. The omega naming convention has become much more popular in daily use, with references to “omega 3” (linolenic oil), “omega 6” (linoleic oil) and “omega 9” (oleic oil) becoming quite common. In other words, an omega 3 FA has its first carbon double bond starting at the third carbon, or an omega 6 FA has its first carbon double bond starting at the sixth carbon, and so on. One other naming convention you might see is something like “18:3”. This takes the form of C:D, where C is the number of carbon atoms in the fatty acid and D is the number of double bonds in the fatty acid. There are several other naming conventions of FAs that you might encounter. Wikipedia has a good summary of what they all mean here.
Below are examples of how the number of carbon double bonds affect shape, and how shape affects the ability of each to pack together. The less able they are to pack together, the more likely they are to stay liquid as the temperature drops, and the opposite is also true – straighter FAs can pack together more densely, and therefore will solidify at warmer temperatures.
Figure 4. Linolenic acid.
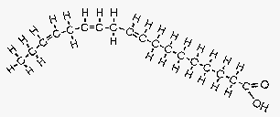
Image courtesy of: http://www.longevinst.org/nlt/newsletter13ext1.htm
Linolenic acid is an example of a polyunsaturated FA. It is an omega 3 FA with three carbon double bonds (very curved), the first occurring at the third carbon atom. This FA is found in flax oil and fish oil which is why flax oil stays liquid in the fridge.
Figure 5. Linoleic acid.
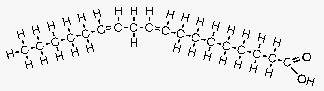
Image courtesy of: http://www.longevinst.org/nlt/newsletter13ext1.htm
Linoleic acid is another example of a polyunsaturated FA. In this case it is an omega 6 FA, with two carbon double bonds (slight bend), with the first occurring at the sixth carbon atom. Note that while this structure may seem similar to the trans fat in Figure 2, the double bonds in this FA are in a “cis” configuration instead of a “trans” configuration. (Note that this model understates the amount of bend present.) Many common vegetable oils contain this FA, which is why they will get cloudy in the fridge but generally remain liquid.
Figure 6. Oleic acid.

Image courtesy of: http://www.longevinst.org/nlt/newsletter13ext1.htm
Monounsaturated FAs have one carbon double bond each, often occurring at either the 9th (omega 9) or 7th (omega 7) carbon atom. Even though these only have one double bond, they have a significantly bent shape. This significant bend is why these long FAs are liquid at room temp, but their length makes them gel at cooler temperatures. Examples of monounsaturated FAs are omega 9 oleic acid found in olive, almond, and avacodo oils, and omega 7 palmitoleic acid found in palm and avocado oils. Above is an example of a monounsaturated (oleic) FA.
Figure 7. Palmitic acid, a saturated FA.
![]()
![]()
Image courtesy of: http://www.longevinst.org/nlt/newsletter13ext1.htm
Finally, above is a saturated fat (completely straight). Found in animal, coconut, palm, and dairy. This straight shape is why these tend to stay solid. So if you want to make a solid type of personal care product such as “butters”, either some saturated fat will be necessary (e.g. coconut), or else some type of wax will have to be used (e.g. beeswax). This example happens to be palmitic acid.
Where Different Types of Fatty Acids Are Found
So, where can these different types of FAs be found? In other words, which plants and animals produce which oils and fats? As a disclaimer, each plant (or animal) source will contain several types of FAs, but it is possible to make generalizations based upon the type of FA predominantly found in a given source. As mentioned, omega 3 polyunsaturated FAs are found in fish oil, flax oil, and to a lesser extent walnuts and walnut oil, which also contain omega 6 FAs. Omega 6 polyunsaturated FAs are found in many “grocery store” vegetable oils, such as corn oil, soy oil, safflower oil, sunflower seeds and oil, and to a lesser extent canola (rapeseed) oil, which also contains monounsaturated FAs. Monounsaturated FAs are found in olive oil, avocados and their oil, and almonds and almond oil. Finally, saturated FAs are found in coconut, palm, milk fat, and animal fat. These are just the most common sources of FAs and is not an all inclusive list. If you are interested in learning about more specific compositions of various FAs from different sources, there are plenty of resources online to answer just about any question.
The types of fats that animals produce tends to be influenced by genetics, but it is also affected by the types of plants the animal eats (in herbivores), or by what the animal’s prey eats (in carnivores). So dietary sources of FAs do have an impact in animal FA composition. A well supported example of this is in cattle and what they are fed. Cattle fed exclusively grass contain more omega 3 FAs, while cattle fed corn and other grains contain much more omega 6 FAs, which can be problematic. (More on this topic in the next section.)
A useful rule of thumb or heuristic regarding plant FAs is that plants have adapted the biochemistry of the lipids they produce based on the temperature of the environment they grow in. Plants need their FAs to remain somewhat malleable, which often means at least some double bond bends. But they have to balance this trade-off with the greater energy efficiency of storing fully saturated FAs. So plants that grow in hot climates such as coconut and palm tend to produce more saturated fats because those are more energy storage efficient, while still being able to stay malleable in hot climates. Plants that live in moderate or Mediterranean temperature climates, such as almonds, olives, or avocados, tend to produce monounsaturated fats because they are more cold tolerant than saturated FAs, but are more energy efficient than the polyunsaturated FAs. Finally, plants that grow in colder climates tend to produce more polyunsaturated FAs, because these are more likely to remain malleable in cold temperatures, despite the drawback that they are less efficient at storing energy. Below is a table that provides a generalized summary of these properties.
| Table 1. Generalized Properties of Plant FAs | ||
| Saturated | Mono | Poly |
| *tropical climate | *Mediterranean climate | *colder climates |
| *no double bonds | *one double bond | *many double bonds |
| *straight | *bent | *very bent or curved |
| *higher melting temp | *moderate melting temp | *low melting temp |
| *most energy stored | *moderate energy stored | *less energy stored |
Interestingly, while warm blooded animals are not subject to the same temperature impacted limitations in the types of FAs they produce that plants are, it seems that fish (which are “cold blooded”) are somewhat subject to this rule of thumb. That is why cold water fish species have greater concentrations of omega 3 polyunsaturated FAs than warm water fish species. So this relationship between temperature and fatty acid type seems to be a rule that biology often uses whenever it doesn’t have other options (like “warm blooded” temperature regulation).
Health Impacts of Different Types of Fatty Acids
Finally, now that we have discussed sources of different FAs, the basics of the different structures of FAs and how that affects their chemical and physical properties, we can touch upon a few of the health effects of different types of FAs. The topic of FAs in human nutrition is actually a huge topic that requires and deserves its own article (or several!). That is not the purpose of this article, so we will only mention a few things here. Again, these are somewhat generalizations, but do hold true most of the time.
From a dietary and health perspective, lipids play numerous roles in the body from hormones, to immune signaling molecules, to energy and energy storage, to insulation, and structure. Lipids comprise a large percentage of cell membranes, including in skin cells.
Omega 3 polyunsaturated FAs and omega 6 polyunsaturated FAs are both “essential” nutrients to the human diet. Human enzymes can make many types of FAs, but the first carbon position that human enzymes can make double bonds is at the 7th omega position. So any biologically necessary FAs that contain carbon double bonds in positions before the 7th carbon must come from dietary sources; thus the importance of making sure you eat the right amounts of Omega 3’s and 6’s – since your body can’t produce these on its own. Strictly speaking, monounsaturated FAs and saturated FAs are not “essential” because the human body can make many (but not all) of them, so they do not have to come from dietary sources. Despite that, consuming certain healthy monounsaturated (e.g. oleic) or saturated fats (e.g. butyric) can have health benefits, even though they are not technically “essential”. In a nutritional sense, “essential” just means that you will die or develop pathologies if you don’t consume enough from your diet. But preventing sickness and death is not quite the same thing as optimal health.
Omega 3 Polyunsaturated (Linolenic)
There is a great deal of medical research literature supporting that omega 3 (linolenic) polyunsaturated FAs have numerous health benefits. In particular, they tend to be anti-inflammatory, improve cell membrane fluidity and permeability, and support brain structure and function. The anti-inflammatory properties of these FAs are particularly important because chronic or inappropriate inflammation plays important roles both in numerous pathologies as well as some of the mechanisms of aging itself. While acute or short term inflammation plays critical roles in fighting infections and in injury, the inflammation should ideally be short term, calm back down (“resolution”), and not linger. It is when inflammation lingers or reemerges chronically that problems occur. And it is this inflammatory biochemical pathway that omega 3 FAs tend to suppress. These traits are true to some extent of alpha-linolenic FAs in general, but are especially true of the FAs Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA). As the models below show, these two FAs have many carbon double bonds and are extremely bent.
Figure 8. EPA has five carbon double bonds. (This diagram uses a shorthand format.)
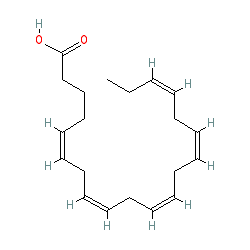
Image courtesy of: http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3362
Figure 9. DHA has six carbon double bonds. (This diagram uses an shorthand format.)
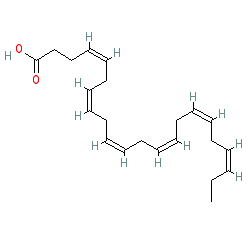 Image courtesy of: http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1051
Image courtesy of: http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1051
Bringing this back to the topic of skin care, the anti-inflammatory effects of omega 3 fats would also be beneficial for the skin. Unfortunately however, fish oil and flax oil do not tend to work well as ingredients in skin care products. Most people don’t fancy the idea of applying products that smell like fish, and as noted above, these types of fats are also very prone to oxidation and spoilage.
Omega 6 Polyunsaturated (Linoleic)
While omega 6 (linoleic) polyunsaturated fatty acids may be essential for human nutrition, consuming too much of them can become quite unhealthy, and they likely contribute to many modern pathologies. This is because omega 6 FA play the opposite role that omega 3 FAs do – they promote inflammation. The medical literature has linked many different types of pathologies to excessive and chronic inflammation, including: cardiovascular disease, autoimmune disorders, cancer, immune senescence, asthma, arthritis, Crohn’s disease, type 2 diabetes, and neurodegenerative diseases such as Parkinson’s and Alzheimer’s. The most concerning thing about this is that over the past 60 years the amount of omega 6 FAs consumed by people in the US and in much of the world has increased dramatically, particularly as they have replaced other sources of dietary fats such as omega 3 and saturated fats. I will leave it to readers to judge for themselves if there is a causal connection between the increase omega 6 consumption and the increase in inflammatory disorders, or if it is merely correlation or coincidence. To conclude about omega 6 FAs, it is important to point out that some omega 6 fats may offer certain health benefits. These include Conjugated Linoleic Acid (CLA) and Gamma Linoleic Acid (GLA). So it is not completely accurate to make gross generalizations about entire classes of fatty acids.
With that said, as someone who develops skin care products, it is important to realize that the skin is also susceptible to damage from chronic inflammation. Therefore, when formulating products it is very important to choose only ingredients that will not contribute to skin inflammation. I bring this up because I have observed some manufactures using low quality omega 6 oils (such as soy oil) as carriers or bases for their products. The only oil we use in our products that is high in omega 6 FAs is grapeseed oil, which we use selectively and are careful to balance it with other oils containing other types of FAs. There are good reasons to use grapeseed oil in skin care, as it provides a natural source of compounds that are beneficial for the skin, including: tocopherols (natural forms of Vitamin E), resveratrol, quercetin, procyanidins, carotenoids, and phytosterols. All of these compounds have benefits for the skin.
Monounsaturated (Oleic)
Like omega 3 fats, monounsaturated FAs such as omega 9 also tend to be anti-inflammatory, although less so than omega 3s. As a result, they are good candidates for both consumption and as ingredients in skin care products. Omega 9 fats have also proven to be cardioprotective, which is part of the reason why “Mediterranean” style diets (which are high in these fatty acids) are correlated with better cardiovascular health.
Saturated Fats
Saturated fats are not necessarily unhealthy, and these have been one of the most unjustly vilified food items (along with cholesterol). In general it is far too simplistic to demonize any entire class of fats. Just as there are beneficial types of omega 6 fats, there are beneficial types of saturated fats. For example, butyric acid, caprylic acid, and lauric acid are all saturated FAs, yet these have research to support various health benefits including positive effects on cancer, type 2 diabetes, inflammatory disorders, antimicrobial activity, and increases in High Density Lipoprotein (HDL) – otherwise known as the “good” “cholesterol”. A general rule of thumb with saturated fats is shorter chain saturated fats tend to be either health positive or health neutral, while long chain saturated fatty acids (e.g. myristic, palmitic) tend to contribute to cardiovascular disease – if consumed in excess. Similar to omega 6 FAs, it is not accurate to make gross generalizations about this entire class of fatty acids.
Cholesterol
Similarly, cholesterol plays essential roles in the body, including digestion (via bile), hormone production (testosterone, estrogen, cortisol, etc.), and maintaining cell membrane fluidity, as it is an essential component of almost all cell membranes. Cholesterol plays a similar role to omega 3 fats in cell membranes because its shape is not particularly straight either. Pathologies involving cholesterol such as cardiovascular disease typically also involve numerous other factors including: chronic inflammation with inappropriate immune activation, high blood pressure, elevated C-reactive protein, elevated homocysteine, excess fibrinogen, elevated blood glucose and insulin, low levels of Vitamins D and K(2), nitric oxide deficiency, and genetic defects of cholesterol metabolism. Therefore solely blaming cholesterol for cardiovascular disease is a gross oversimplification. The science-based Life Extension Foundation has compiled a good summary on this topic here.
For clarification, LDL (Low Density Lipoprotein) and HDL (High Density Lipoprotein) are not actually cholesterol themselves, they are protein carriers of cholesterol used as surrogates to measure cholesterol. LDL carries cholesterol from the liver to the cells of the body, while HDL carries cholesterol from the cells of the body back to the liver. You can think of them like buses carrying passengers from home to work (LDL) or from work to home (HDL). Measuring LDL and HDL is like measuring the number of buses to determine the number of people moving around.
Trans Fats
Trans fats on the other hand, are universally unhealthy FAs that very rarely occur in nature. These are 98% synthetic man made (via artificial hydrogenation). The artificial trans (rather than cis) position at the carbon double bonds alters the shape of the FA that creates important functional differences. These functional differences have been shown to contribute to cardiovascular disease, stroke, type 2 diabetes, cancer, increased inflammation, and infertility. Manufacturers use trans fats because they are inexpensive, easy to produce and work with, and last a long time. So these are good for manufacturers, but bad for consumers. One of our philosophies at Nature’s Complement is that we will never choose an ingredient for the sake of cost or ease of manufacture if it negatively affects the health properties of the product. That is just an unethical practice that has grown unfortunately too common.
Herbicides, Pesticides, and Fungicides, Oh My
One final topic I want to mention that gets little attention is the potential health effects that herbicides, pesticides, and fungicides may have on plant lipids. While some of these chemicals are water soluble and will wash away and end up in the water table (which is not necessarily good either), many of these synthetic petroleum-based chemicals are lipid soluble, and are hydrophobic (water insoluble), so they may not wash away with rain or irrigation. Since they often stick around (literally), they are likely to remain on or near the plants, and therefore are likely to be harvested with the plants. In the case of plants produced for oils or fats this is even more concerning than those produced for other components like vegetables. For example, if a farmer sprays such chemicals around their olive or almond trees and then the almonds and olives are harvested and pressed into oil, such lipid soluble chemicals are more likely to end up in the final oil product than they would with a water based product like most vegetables. And many of these chemicals are quite harmful to human health. This is why Nature’s Complement uses ingredients from organic sources produced without such chemicals whenever possible. I will be writing more on the topic of herbicides, pesticides, and fungicides in a forthcoming book review.
Conclusion
This article has covered a lot of ground, but is only an introduction to the vast knowledge that is available on the topic. I wanted to cover some of these basics as there is a great deal of mis-information that can be confusing, and having a good understanding of the basics on this topic, will allow for a reader to develop a fact-based view of the topic. With that said, there is a related topic that is even more important when it comes to skin care and Nature’s Complement products: how do these various types of FAs interact with the skin? What different effects do different types of FAs have on the skin? We have already mentioned potential inflammatory or anti-inflammatory effects, but there are many other important biochemical and physiological considerations including, antioxidant effects, barrier protection from the environment, importance to skin structure composition, importance to wound healing, potential affects related to lipofuscin and glycation, affects on pathways related to aging, and others. These topics will be covered in the future, so keep an eye out, or subscribe to our newsletter to receive notification when new articles are posted. In the mean time, I need to go cut some soap.
For Health,
Rob
References Cited: Please note, this article was written from memory, so I do not have exact page citations. However, I do have a list of textbooks that I still reference from college. The ones relevant to this article are listed below:
Nelson DL, Lehninger AL, Cox MM. Lehninger Principles of Biochemistry. Macmillan; 2008.
Campbell & Farrell, Biochemistry, 6th edition. Thomas-Brooks / Cole; 2008.
Gropper S, Smith J. Advanced Nutrition and Human Metabolism. Cengage Learning; 2008.
Murphy KM, Travers P, Walport M. Janeway’s Immunobiology, 7th edition. Garland Science; 2007.
Neil A. Campbell, Jane B. Reece. Biology. Pearson, Benjamin Cummings; 2005.
McMurry, John E., Organic Chemistry, 6th edition. Brooks Cole; 2003.
Don’t forget! If you decide to buy books online you can support our web site by buying them through our Amazon affiliate links by clicking the images below.
Nature's Complement is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program. If you purchase products on Amazon through any of our affiliate links, we get a small percentage of the transaction, at no extra cost to you. We spend a lot of time writing the articles on this site, and all this information is provided free of charge. When you use our affiliate links, you support the writing you enjoy without necessarily buying our products. (However we would appreciate if you would do that too!) Thank you for helping to support our work, however you choose to do so.
These statements have not been evaluated by the Food and Drug Administration. This information and/or products are not intended to diagnose, treat, cure or prevent any disease.



[…] don’t even need to leave our region to find one of our favorite skin-care vendors going deep into the science behind fats. One of the reasons we love sharing all this hard work people have put into explaining and […]
[…] of lipids are best used for what type of product? We covered this to some degree in our article “Understanding Oils & Fats For Health And Beauty”. What ingredients are safe to stabilize the product (if any). What ingredients can I add to make […]